- Co Telsan 40/12.5 Tablet contains
Telmisartan 40mg
Hydrochlorothiazide 12.5mg - Co Telsan 80/12.5 Tablet contains
Telmisartan 80mg
Hydrochlorothiazide 12.5mg
Co-Telsan is indicated for the treatment of hypertension.
How Co-Telsan Tablet Works?
- Telmisartan
Telmisartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin 2 by selectively blocking the binding of angiotensin 2 to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin 2 synthesis. - Hydrochlorothiazide
Belonging to the thiazide group of diuretics, Hydrochlorothiazide affects the renal tubular mechanisms of electrolyte re-absorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of Hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin 2, so co-administration of an angiotensin 2 receptor antagonist tends to reverse the potassium loss associated with these diuretics.
When to avoid Co-Telsan Tablets?
Co-Telsan is contraindicated in patients who are hypersensitive to any component of this combination. The combination of Telmisartan and Hydrochlorothiazide is also contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
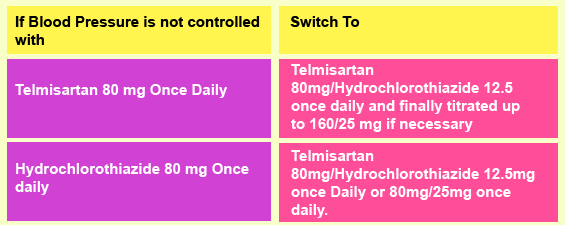
Recommended Dosage of Co-Telsan Tablets
Therapy with Co-Telsan Tablets should be started when Telmisartan monotherapy does not give the desired result. Co-Telsan may be co-administered with other antihypertensive agents and this drug is administered irrespective of food.
Dosage of Co-Telsan in Renal Dysfunction
Co-Telsan Tablets should be used in the patients with creatinine clearance of >30 ml/min. In patients with creatinine clearance of <30 ml/min, loop diuretics should be given instead of thiazides.
Dosage of Co-Telsan in Hepatic Dysfunction
Co-Telsan Tablets should not be given to patients with severe hepatic dysfunction. If the physician decides to give the combination to patients with biliary obstructive disorders or hepatic insufficiency, Co-Telsan 40mg/12.5mg should be given under strict observation.
Side Effects of Co-Telsan
Co-Telsan side effects are comparable to placebo. Most side effects are mild and transient and do not require discontinuation of therapy.
- The side effects reported at a rate less than 2% in patients treated with Co-Telsan Tablets and at a greater rate than in patients treated with placebo were back pain, dyspepsia, vomiting, tachycardia, hypokalemia, bronchitis, pharyngitis, rash, hypotension postural and abdominal pain.
- The side effects reported at a rate of 2% or greater in patients treated with Co-Telsan, but were as, or more common in the placebo group were pain, headache, cough, urinary tract infection. Adverse events occurred at approximately the same rates in men and women, older and younger patients, and black and non-black patients.
- In controlled trials, 0.3% of patients treated with Co-Telsan discontinued due to orthostatic hypotension.

Leave A Comment